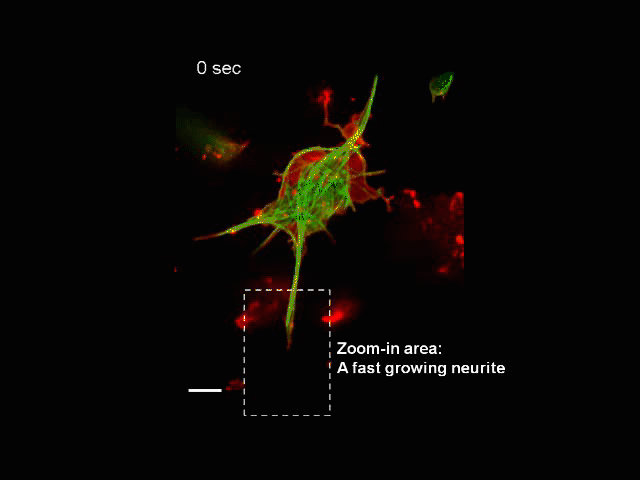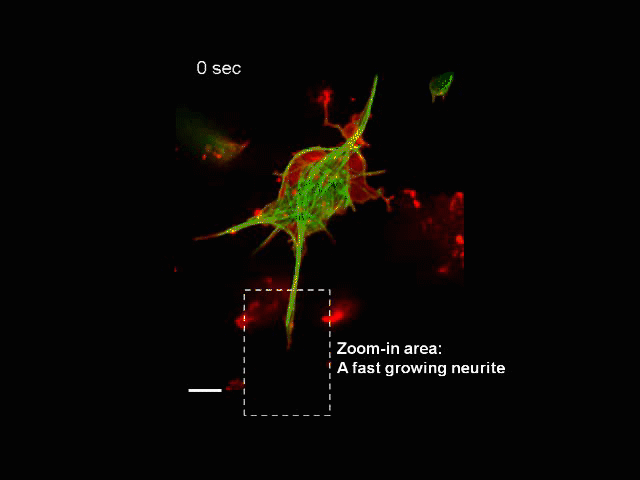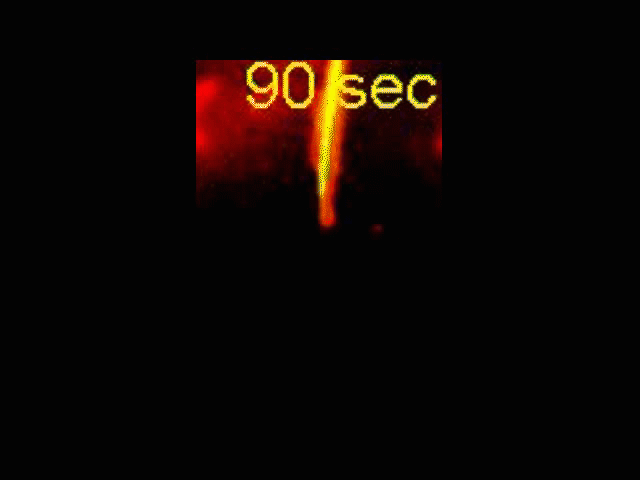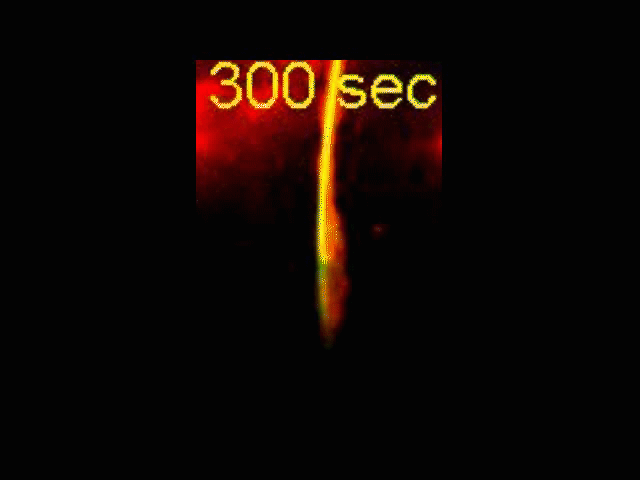
Neurite Outgrowth
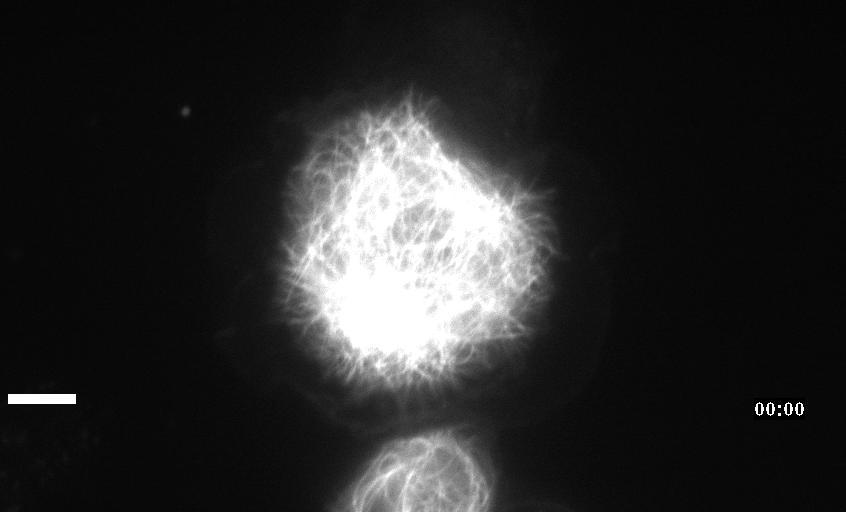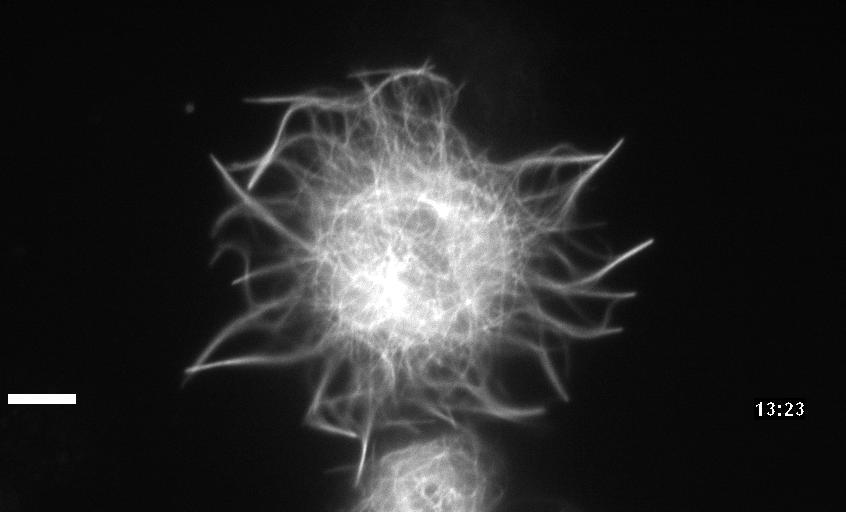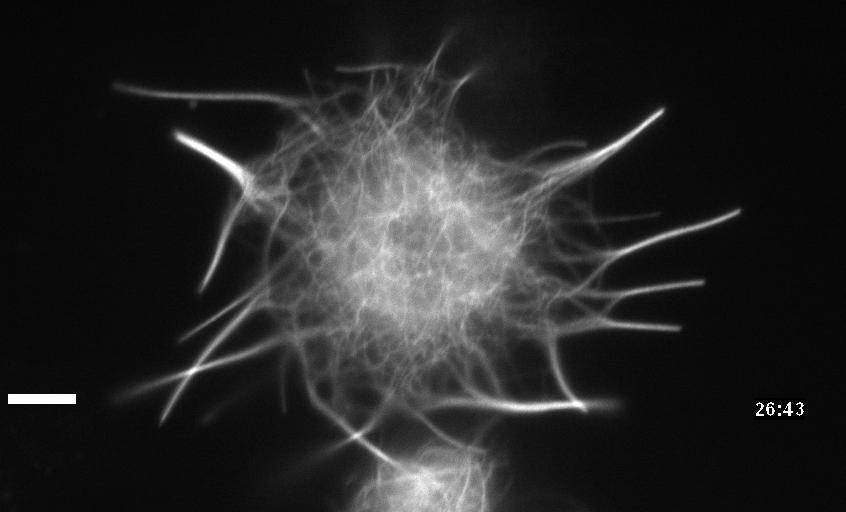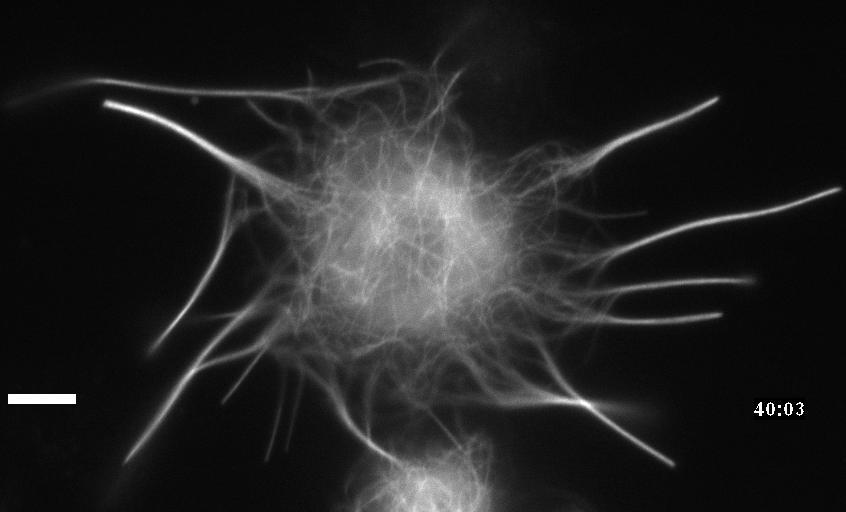
Neuronal development involves a dramatic reorganization of the microtubule cytoskeleton. This facilitates the formation of highly polarized neurons, extending axons and dendrites, from non-polarized neuronal precursors and is therefore the subject of much study. In the search for mechanisms underlying microtubule remodelling during neuronal development, we uncovered a role for the molecular motor kinesin-1 (Barlan et al., 2013; Jolly et al., 2010; Lu et al., 2013). Kinesin-1 ‘slides’ microtubules against each other to drive initial neurite formation (Lu et al., 2013). Kinesin-1 can achieve this by binding microtubules at both ends of the motor, and loss of the non-motor domain microtubule binding site decreases sliding and results in severely interrupted neurite outgrowth in vivo (Winding et al., 2016). As well as neurite outgrowth, microtubule sliding also occurs during neurite regeneration (Lu et al., 2015).
In addition to kinesin-1, we have also identified a role for the ‘mitotic’ kinesin, kinesin-6 (KLP-Pav) in microtubule sliding. Pavarotti counteracts the action of kinesin-1 to inhibit sliding. Loss of this motor results in overextended and mistargeted axons in vivo (del Castillo et al., 2015) demonstrating the need for proper regulation of microtubule sliding to correctly tailor neurite outgrowth. We were therefore also interested in studying factors regulating Pavarotti’s ability to inhibit microtubule sliding. We have recently identified a role for the kinase Tricornered (Trc) – known to phosphorylate Pavarotti during mitotic progression – in regulating microtubule sliding in neurons and neurite outgrowth in vivo (Norkett et al., 2019).
We are also interested in understanding how microtubules are sort in neurites. We observed that in the very early stages of neuron development, the developing axons contain microtubules of mixed polarity. However, by the later stages, the microtubules become uniform with all the plus-ends directed outwards. We showed that dynein recruited to cortical actin is responsible for this organization as it pushes the minus-end-out microtubules out of the axons (del Castillo et al., 2015). The next challenge is to understand how dynein is attached to the actin scaffold and why it rearranges microtubules in axons, but not in dendrites.